2na S 2h2o L 2naoh Aq H2 G
2Nas 2H2Ol---- 2NaOHaq H2g 184 grams. What mass of phosphorus will be needed to produce 325 mol.
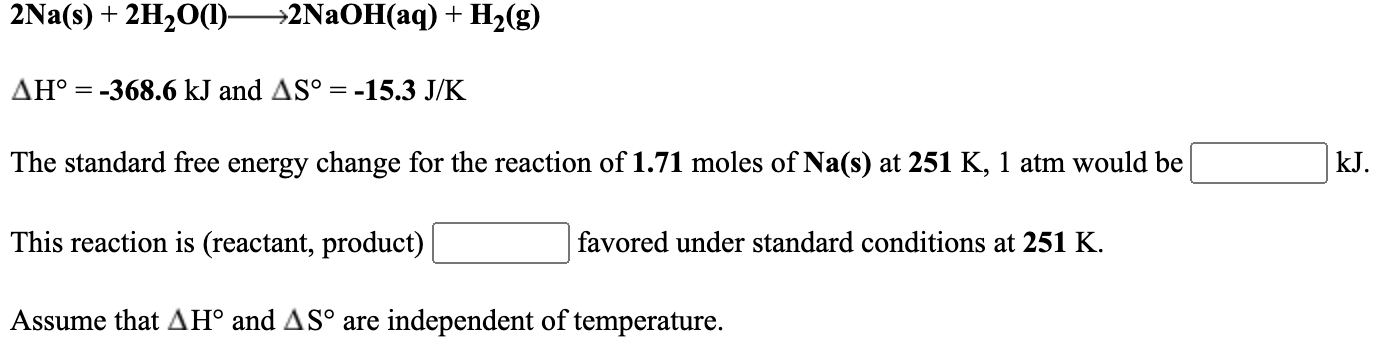
Solved 2na S 2h2o 1 2naoh Aq H2 G Ah 368 6 Kj Chegg Com
For the reaction 2Na s 2H2O l ----- 2NaOH aq H2 g delta H -3686 kJ and delta S -153 JK The maximum amount of work that could be done when 247 moles of Na s react at 295 K 1 atm is______.

. 2Nas 2H2Ol---- 2NaOHaq H2g 8 moles. Find your perfect car with Edmunds expert reviews car comparisons and pricing tools. How many grams of sodium will react with water to produce 40 mol of hydrogen in the following reaction.
Option C Explanation 2Na s 2H 2 O l 2NaOH aq H 2g Relative molecular mass of NaOH 23 16 1 40smol-1 2 23g of sodium react with water to produce 2 40g of NaOH. In the past Henry has also been known as Henry G Morgan Morgan Henry and Henry G Attorney. It has been oxidized.
Scotch Plains NJ United States. 20 PbSO4s 2 H2Ol充电时阳极PbSO4s 2 H2Ol -2e PbO2s 4H aq SO42- aq 阴极PbSO4s 2e Pbs SO42- aq 9氢氧燃料电池P77总反应2H2 O2 2H2O酸性条件负极 2H2 -4e- 4H 正极O2 4H 4e- 2 H2碱性条件负极2H2 4OH -4e- 4 H2O 正极O2 2H2O 4e- 4OH10. 2Nas 2H2Ol---- 2NaOHaq H2g 403 grams.
2Na s 2H 2 O l 2NaOH aq H 2 g Double Replacement or metathesis Reaction. It has released and electron. Up to 256 cash back Get the detailed answer.
A redox reaction. As the oxidation state has decreased the element was reduced. Henry Morgan currently lives in Livingston NJ.
Earned the Home Brewed Goodness Level 3 badge. In the past Henry has also lived in Hope NJ and Greendell NJ. Henry Morgan was born on 12141920 and is 101 years old.
Sodium reacts with water to form sodium hydroxide and hydrogen gas. How can we tell that it has reduced if. Phosphorous burns in air to produce a phosphorus oxide in the following reaction.
Aq Imagings practice location is listed as. Please show each step so that I may understand it better thanks in. The balanced equation which represents the above reaction is.
What mass of phosphorus will be needed to produce 325. Contact Personal Details. 1921 Oak Tree Rd Edison NJ 08820-2073 and can be reached via phone at 732 662-1831.
Aq Imagings NPI Number is 1043737802 and has been listed in the NPI registry for 4 years. Phosphorous burns in air to produce a phosphorus oxide in the following reaction. How many grams of sodium will react with water to produce 40 mol of hydrogen in the following reaction.
Aq Imaging is a Diagnostic Radiologist taxonomy code 2085R0202X located in Edison New Jersey. 2Nas 2H2Ol---- 2NaOHaq H2g 184 grams. 2Nas 2H2Ol 2NaOHaq H2g In this chemical equation H2O is the oxidizing agent.
This problem has been solved. Its still overcarbed but after it sits and breathes its pleasant. Single Replacement or substitution Reaction.
Assume that delta H and delta S are independent of temperature. What type of reaction is. So it won electrons.
H from water has gained an electron so it changed the oxidation state from 1 to 0. Save up to 3030 on one of 2 used HUMMER H2s in Avenel NJ. 2Nas2H₂Ol2NaOHaqH₂g Sodium changes from 0 oxidation state to 1.

Which Is The Oxidising Agent In The Following Equation Haso2 Aq Sn 2 Aq H Aq As S Sn 4 Aq H2o I
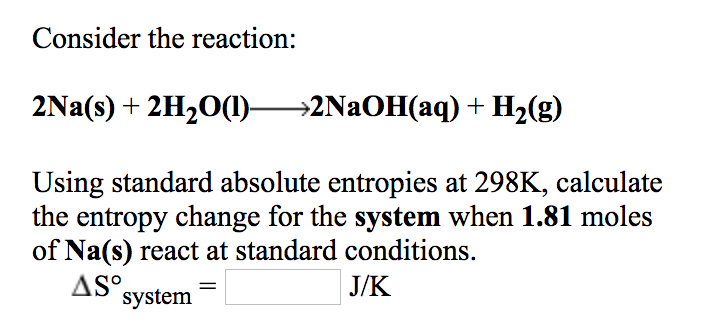
Solved Consider The Reaction 2na S 2h2o 1 2naoh Aq Chegg Com

Solved Consider The Reaction 2na S 2h2o L 2naoh Aq Chegg Com

Assign An Oxidation Number To Each Atom In The Products 2na S 2h2o L 2naoh Aq H2 G Assign An Brainly Com
No comments for "2na S 2h2o L 2naoh Aq H2 G"
Post a Comment